Aluminum is a metal of great utility and demand; it is strong, lightweight, and corrosion-resistant. Aluminum surfaces develop unattractive oxidation- chalky and discolored-when left too long under air, moisture, and other environmental purveyors. Whether restoring aluminum cookware, vehicle parts, outdoor furniture, or industrial equipment, grasping the necessary knowledge regarding the removal of aluminum oxidation to its full capacity is crucial to preserve its appearance and utility. This guide presents tried and tested methods for aluminum cleaning and polishing, offering step-by-step insights, professional wins, and recommended reforms. By the end of this article, one will possess the knowledge capable of transforming dreary aluminum oxides into sleek and polished surfaces that yield long-term results.
The Science of Aluminum Oxidation

Aluminum oxidation constitutes aluminum reacting with oxygen from the environment to form a thin and protective coating of aluminum oxide on its surface. In contrast to rust in iron, which causes further corrosion, this oxidation actually prevents corrosion, since the oxide layer is extremely stable towards environmental aggressions. Interestingly, the layer may over a period of time become an excellent adhesion base for the accumulation of dust, staining particles, or even a new layer of oxidation, giving the appearance of a surface being dull or irregular. An understanding of this process is essential in selecting from the great variety of cleaning methods that can remove buildup while leaving the valuable oxide layer intact.
Understanding Aluminum’s Properties
Lightweight
Aluminum has a low density of 2.7 g/cm³, about one-third as heavy as steel. This property makes it an appropriate material for elite applications where weight reduction is top priority under transportation or aerospace.
High Strength-to-Weight Ratio
While lightweight, aluminum alloys gain a high strength-to-weight ratio that imparts resistance to considerable mechanical stresses and remains relatively blue-black light in weight.
Corrosion Resistance
By the very nature of it, aluminum undergoes the formation of thin oxide film almost immediately on the surface when in contact with the air. This oxide layer becomes highly resistant to corrosion, thereby guaranteeing the durability of aluminum even in a harsh environment, including marine atmosphere.
Thermal Conductivity
Heat transfer in aluminum is quite high at about 235 W/m·K. Response to heat strengthening is beneficial for heat exchangers, engine components, and cooling systems.
Electrical Conductivity
Though lagging behind copper in terms of electrical conductivity, aluminum possesses good electrical conductivity at about 61% that of copper. It is used extensively in power transmission and distribution systems on account of its light weight and cheapness.
Malleability and Ductility
Aluminum offers good workability and can be molded, pressed, or stretched into an array of shapes without breaking. Thus, it is suitable for the fabrication of extrusions, and rolled, forged products.
What Causes Aluminum to Oxidize?
Aluminum oxidation occurs when aluminum reacts with oxygen in its environment and deposits a thin coat of aluminum oxide onto its surface. This means of protection through rapid oxide layer formation is called passivation and occurs almost at the instant the metal surface absorbs air or moisture. The oxide layer strongly binds with the aluminum surface and acts as an effective barrier against any sort of corrosion. The rate of oxidation may increase if the aluminum surface gets exposed to high humidity, high temperature, or saltwater. However, unlike iron rust, aluminum oxide does not peel off, thereby allowing it to increase the strength of the metal for resisting further destructive interaction against the environment over a period of time.
The Role of Environmental Factors in Oxidation
Then, environmental conditions play a very important role in deciding the rates and extent of oxidation of various materials. For example, a high humidity environment enhances the availability of water molecules needed for an oxidation reaction; it also aids in the breakdown of certain protective oxide films on metals such as copper or zinc. Higher temperature increases the kinetic energy of the reactant molecules, helping them to overcome oxidation faster. Also, pollutants should be able to fulfill their role of acid deposition. Such acid deposition greatly enhances corrosive effects on any exposed surface.
In coastal areas, chlorides threaten especially those corrosion inhibiting metals like aluminum and steel. Chloride ions aggressively attack oxide layers, resulting in localized pitting and loss of structural integrity under the influence of varying temperature cycles. Advanced knowledge emphasizes the criticality of material design and protective coatings to counteract these effects and thereby extend the service life of metals under harsh environmental exposure.
Identifying Oxidized Aluminum

Oxidized aluminum is dull whitish; that is the indication of aluminum oxide being formed on its surface. The layer generally feels powdery and may not show any metallic sheen commonly seen in untreated aluminum. Sometimes aluminum surfaces after oxidation may show a slight discoloration and roughness. In many instances, simple eye inspection would be enough, yet aluminum can simply be measured using chemical testing or microscopy to detect the actual thickness of the oxide layer.
Signs of Oxidation on Aluminum Surfaces
Several environmental and material-related factors influence the rate and extent of oxidation of aluminum. Moisture and temperature produce significant effects; such environmental conditions stimulate the oxidation by making water molecules available and providing thermal energy. Likewise, industrial pollutants such as sulfur dioxide or chlorides increase oxidation rates by introducing chemically aggressive species at the surface. At a material level, the purity of aluminum governs directly how susceptible it is, for commercial alloys containing additives usually provide varying responses-increasing or decreasing-species with the rate of oxidation and resistance. Interpreting these influencing factors becomes critical in predicting and combating the incidence of oxidation with aluminum’s performance and life.
Differentiating Between Oxidation and Tarnish
| Parameter | Oxidation | Tarnish |
|---|---|---|
| Definition | Reaction with oxygen forming a surface layer. | Surface corrosion due to sulfur or other compounds. |
| Appearance | Dull or whitish layer. | Black, gray, or yellow discoloration. |
| Cause | Exposure to oxygen and moisture. | Reaction with sulfur, chlorides, or pollutants. |
| Material Affected | Metals like aluminum, steel, or copper. | Primarily silver and copper-based alloys. |
| Protection Mechanism | Often creates a protective passive layer. | No protective role, purely corrosive. |
| Chemical Process | Oxidation-reduction reaction. | Sulfide or halide chemical interaction. |
| Effect on Durability | Can reduce aesthetics but protects metal. | Degrades and weakens material over time. |
| Removal Method | Abrasive cleaning or chemical treatment. | Polishing or chemical tarnish removers. |
| Preventative Measures | Anodizing or protective coatings. | Anti-tarnish solutions or protective storage. |
Assessing the Severity of Oxidation
Based on these essential parameters, the severity of oxidation on a material is measured by colorimetric changes on the surface, structural integrity, and deep penetration. Quantitative determination by measuring the oxide composition, surface morphology, and cross-sectional damage can be conducted by using X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), etc. Also, under environmental conditions, electrochemical tests such as cyclic voltammetry can be used to analyze the footprint and oxidation rate.
Exposure level plays an important environmental role: materials in situations in which high humidity or salinity prevails undergo oxidation at a much-pronounced rate owing to their electrolytic activities. Materials seem to be more susceptible to oxidation, such as iron and carbon steel, unless it is coated or otherwise treated. In general, data reveal better resistance offered by stainless steel alloys with relatively high amounts of chromium, wherein a passivation layer is formed that reduces oxidation. Under a higher and salted environment with stainless steel, high resistance corrosion monitoring and performance evaluation are conducted. For sure, these must be endorsed with regular inspection and test protocols, dependent on the specific material used in the oxidized metal.
Methods to Remove Oxidation from Aluminum

Using White Vinegar
White vinegar acts as a great solution to aluminum oxidation: use this liquid by rubbing some on the oxidized area, allowing it to work its magic for about a few minutes, gently scrubbing with a soft brush or cloth, then finally rinsing the area well with plain water to avoid residue formation.
Fine Sanding
A light buffing method can work wonderfully for stubborn oxidation removal-a little bit of sanding with fine-grit sandpaper will do wonders in restoring the aluminum surface. Just be sure to sand evenly to prevent scratches or, most definitely, uneven surfaces.
Commercial Aluminum Cleaners
Aluminum cleaners dissolve cancellations and protect the metal. Follow instructions for the specific product for safe and efficient application.
Baking Soda Paste
Make a thick paste with baking soda and water and apply it on the oxidized aluminum. Scrub very gently with a non-abrasive pad, rinse very well, and dry to complete the restoration workshop.
Step-by-Step Guide for Small Aluminum Surfaces
- Preparation
Only after having on hand the soft cloth, non-abrasive scrubber, baking soda and water, and mild dish soap should one prepare the aluminum surface for gentle rinsing from any loose dirt or debris. - Cleaning the Surface
Now, mix up your warm water and mild dish soap in a ratio of about 4:1. Use your soft cloth to wash the aluminum surface, applying light pressure to work out the grease and grime. Once done, rinse that surface well with clean water to give it a good wash-off from the soap. - Addressing Oxidation
Next, for those spots that really show in oxidation, bring out your baking soda paste. Mix three parts baking soda with one part water, until thick. Apply that paste generously to all areas showing oxidation, and let it sit for a good few minutes. - Scrubbing Gently
Use a non-abrasive scrubber to softly rub the paste into the aluminum surface in circular motions. Do not press hard to avoid damaging the aluminum surface. Use a soft-bristle brush for complex surfaces to reach into crevices. - Rinsing and Drying
Rinse off the baking soda paste thoroughly by pouring clean water all over the aluminum surface. After rinsing, use a clean microfiber cloth to pat-dry the surface to prevent water spots. - Final Polishing (Optional)
Then polish with a commercial aluminum polish for the best finish. Apply the polish according to the directions on the bottle for a transparent, evenly strong shine.
Techniques for Large Aluminum Surfaces
The preparation phase, along with the actual work on aluminum staining, is crucial for a smooth and professional finish on large aluminum surfaces. Clean the entire working surface very well in warm water along with mild detergent before staining to get rid of dirt, grease, or any other contaminants. The actual uniform abrasion phase can be well taken care of by orbital sanders or special aluminum-cleaning pads for large areas, so that uneven wear traces are minimized could result from manual abrasive methods.
Having been cleaned and prepared, the aluminum is now ready for inspection of oxidation or corrosion sites. Commercial-grade aluminum brighteners and deoxidizers work instantly well on these areas. Apply the solution evenly, following product instructions to ensure good coverage and to prevent damage to the aluminum structure.
Use power buffers for polishing that are considered to be of the highest specification, fitted with non-abrasive pads; this reduces manual exertion whilst laying the polish in an even manner. Maintain accuracy and avoid streaking across wide surfaces by working in sections from one edge with overlapping passes. Sealing the aluminum with industrial-grade sealant as the final procedure will guard against weather conditions exposure, hence fostering the durability of the aluminum whilst maintaining its polished look through time.
Using Chemical Solutions vs. Mechanical Methods
| Key Point | Chemical Solutions | Mechanical Methods |
|---|---|---|
| Ease of Use | Simple application process | Requires physical effort and precision |
| Time Efficiency | Faster due to chemical action | Time-consuming for large surfaces |
| Material Compatibility | May corrode sensitive materials | Gentle when correct tools are used |
| Environmental Impact | May produce harmful waste | Minimal environmental impact |
| Cost Effectiveness | Can be costly depending on product quality | Typically requires one-time equipment investment |
| Skill Requirement | Minimal skill required | Requires proper training for effective results |
| Surface Finish | Can leave residue or streaks | Achieves high-quality, uniform finish |
| Safety Concerns | Risk of chemical burns or inhalation | Risk of mechanical damage if improperly used |
| Durability of Results | Temporary unless sealed | Long-lasting with proper execution |
| Maintenance | Requires periodic reapplication | Low maintenance once completed properly |
Recommended Tools and Materials
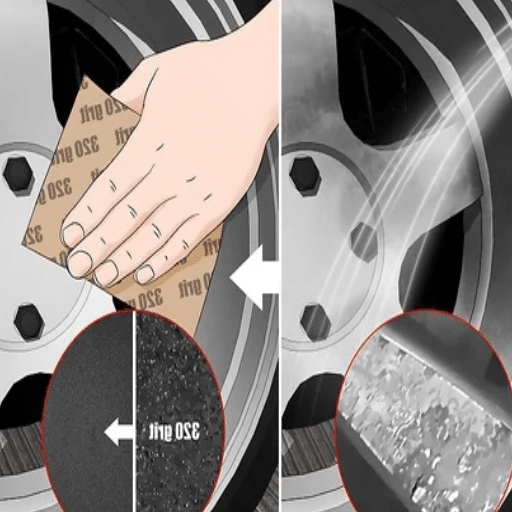
Tools and materials for the very best results are:
- Protective Gear (gloves, goggles, and a respirator) to keep the user safe during application or operation.
- An appropriate Cleaning Agent for the material or surface treated.
- Abrasives of top quality such as sandpaper and polishing pads compatible with a fine smooth surface finish.
- Application Equipment: What works best here depends on the particular material and project size: brushes, rollers, and sprayers to name a few.
- Sealing Products: If and when applicable to augment lifespan and upkeep of the finish of the treated surface.
- Measuring equipment for precision, where specific proportions or thicknesses are required.
These tools and materials guarantee efficiency, safety, and quality.
Essential Tools for Removing Oxidation
Any act of removal of oxidation demands a host of tools capable of targeting the buildup without traumatizing the exposed surface beneath it. Here are a few crucial tools for the job:
- Orbital Polishers – Operate by means of ambiguous circular motion gently and evenly to remove the oxidation from metals, automotive paints, or fiberglass. Their precision acts to minimize uneven wear to unnecessary surface damage.
- High-Quality Compound Pads – These are essential for polishing compounds, which guarantees an even application with the required abrasiveness to remove from the surface all layers of oxidation.
- Oxidation Removal Compounds – Chemically engineered to breakdown oxidation and bring about restoration of clarity and gloss to affected areas. It is of utmost importance to select a compound that is compatible with the substrate material.
- Microfiber Cloths – Used in finishing stages for buffing away excess residue from polishing compounds. Their soft texture will not cause scratching and assures a clean finish.
- Surface Preparation Cleaners – Cleaning all possible contaminants before the oxidation treatment assists in increasing the adhesion of post-treatment protective coatings.
Consistency in applying these tools will result in increased oxidation removal plus treated surface strength and durability. Proper techniques are a must with the right tools to produce professional results.
Recommended Cleaning Products and DIY Alternatives
Commercial Cleaning Products:
- 3M Marine Aluminum Restorer and Polish
An aluminum oxidation remover has been specially formulated, along with other marine-grade metals, giving it an ability to restore the shine while acting as a protective barrier from corrosion into the future. - Bar Keepers Friend Soft Cleanser
Famous for the versatility, it removes oxidation, stubborn rust, and mineral deposits. Being non-abrasive, the formula goes for metals, glass, and even porcelain. - Meguiar’s M4916 Marine/RV Heavy Duty Oxidation Remover
Designed for restoring heavy oxidation using diminishing abrasive technology, it polishes gently for the restoration of clarity and gloss.
DIY Alternatives:
- Vinegar and Baking Soda Solution
When mixed, vinegar and baking soda offer natural cleansers that are excellent in breaking down the oxidation and grime. This solution works perfectly for smaller outbreaks and environmentally friendly approaches to cleaning! - Lemon Juice with Salt
A paste made from lemon juice and salt provides a mild acid and abrasive action for treating slight oxidation or tarnish on metal surfaces. This treatment can work wonders on brass or copper items. - Aluminum Foil and Water for Rust Removal
Aluminum foil dipped in water will remove light rust and oxidation through gentle abrasion developed from the chemical interaction between the foil and oxidized metal.
Safety Precautions During the Cleaning Process

Important Safety Guidelines
- Wear Protective Gear: Always use gloves, goggles, and masks to avoid skin contact, eye irritation, or inhalation of harmful substances during cleaning.
- Ensure Proper Ventilation: Cleaning is done in the environment with good ventilation to diminish exhaust from an agent or chemical reactions.
- Avoid Mixing Chemicals: Never combine cleaning products, such as bleach and ammonia, as this can create toxic gases.
- Test on a Small Area: Prior to dropping any type of cleaning solution on your object, be sure to test it on a tucked-away hidden spot, just to be sure nothing unsightly happens.
- Follow Manufacturer Instructions: Adhere strictly to all instructions and specifications laid out in the product documentation, so as to avoid any untoward accidents or unsavory events.
Protective Gear: Gloves and Safety Goggles
To be safeguarded against exposure and hazards, one really must put on proper protective equipment when handling chemicals or conducting cleaning procedures. Nitrile or neoprene gloves particularly provide vast resistance to most chemicals and thus prevent the potential risk of having irritated skin or chemical burns. Goggles are another PPE indispensable for protecting eyes from harmful fumes, splashes, and debris, many nowadays have anti-fog coating and good sealing to maintain clear vision and comfortable fit during extended usage. A proper combination of gloves and goggles has been established through studies to greatly lessen the chance of injuries, hence every person working with hazardous materials is advised to use them. Always do an inspection for any faults on a protective gear before use and discard it should it exhibit any sign of wear or damage.
Importance of Working in a Well-Ventilated Area
Considerable concern is directed to ensuring proper ventilation within the working environments for safety and efficiency. Poorly adverse environment accumulates hazardous substances such as Volatile Organic Compounds (VOCs), airborne particles, or hazardous/odorous fumes that may carry health concerns of serious nature toward workers over time. According to the accepted standard for occupational safety, airing/code sufficiently controls contaminant concentration level, hence minimization of respiratory condition, whilst considering better commercial air-conditioning. Airports’ exhaust system, like fume hood(s) or high-efficiency particulate air (HEPA) filters, will be recommended when working with chemicals or dust-producing equipment. Efficient ventilation also regulates temperature and humidity, forming a comfortable workplace that is well-compliant. Integrating a proper ventilation strategy within an organization will guarantee its conformity to regulations on health and safety and boost operational efficiency.
Reference Sources
-
- Key Findings: This study explores the self-organization of anodic aluminum oxide (AAO) surfaces, focusing on their mixed oxide-hydroxide composition. It provides insights into the structural and chemical properties of anodized aluminum.
-
- Key Findings: Highlights the importance of oxide layer removal in laser welding processes to improve joint quality and mechanical properties.






