Considered as the world’s common metal, aluminum is respected for being light, tough, and corrosion resistant. Yet many wonder if aluminum is susceptible to the very familiar types of deterioration, like rust, or if it succumbs to some other form of chemical reaction occurring over a period. This article provides an in-depth explanation of the science behind the resistant nature of aluminum, which prevents it from rusting in the same way steel or iron does, instead undergoing a unique process of oxidation.
The Importance of Understanding Aluminum Corrosion

If corrosion is set to be a player, then it is a strong factor in how aluminum will remain, perform, and serve in an environment. While aluminum forms an oxide film by itself, which is considered a protective layer against corrosion, under certain conditions, the said film can be attacked and the aluminum starved away.
Things such as prolonged in-the-wild chlorides, extremes of pH range, and galvanic corrosion when aluminum is in contact with other metals act most proficiently in accelerating corrosion. It is upon this knowledge that we can know the vulnerabilities and utilize it in the selection of alloys, surface treatments, or coatings that can assure the durability of aluminum.
Why is Aluminum Widely Used
Aluminum’s widespread use can be explained from the integration of properties that are so requisite in other industries. Here are the key advantages:
- High strength-to-weight ratio: Aluminum being a naturally light material is very hard, thus applied in aerospace, automotive, and construction industries
- Excellent corrosion resistance: Forms a natural oxide film when exposed to air
- Superior conductivity: High thermal and electrical conductivities make it ideal for power transmission and heat exchange applications
- Exceptional recyclability: Almost 75% of all aluminum ever produced is still in use, consuming just 5% as much energy to recycle as to produce originally
- Versatile alloy development: New alloys make aluminum adaptable for specialized applications including high-performance composites and renewable energy components
Impact of Corrosion on Aluminum Products
Barring an aggressive environment, where corrosion incidents threaten a successful application, the natural resistance of aluminum products imparted to them by their oxide layer remains a pleasant factor. However, certain conditions can compromise this protective layer:
Environmental Threats
- Highly saline environments coupled with moisture
- Low pH or acidic environments
- Industrial pollutants
- Chloride concentration in marine and coastal environments
Types of Aluminum Corrosion
- Pitting corrosion: The most prominent form, characterized by small, very deep pits that weaken aluminum components over time
- Galvanic corrosion: Occurs when aluminum is in physical contact with metals of a more noble nature under corrosive environments
Solution: Surface treatments and coating processes such as anodizing, powder coating, and corrosion-resistant alloys enhance aluminum performance in aggressive applications across industries from aerospace to construction.
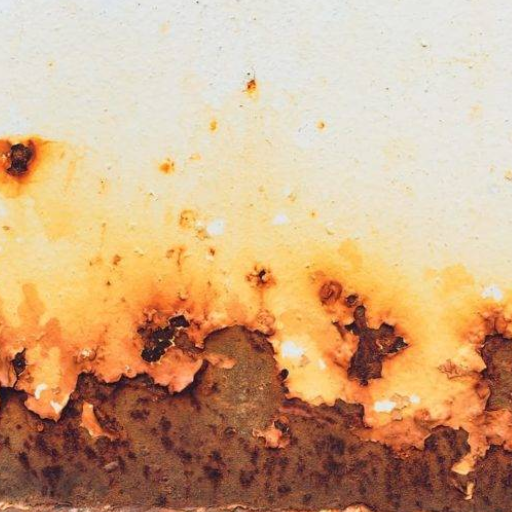
Does Aluminum Rust or Corrode?

It does not rust, because rust is a term used when corrosion of iron or steel occurs due to oxidation. Aluminum, however, can suffer corrosion. Unlike rust, corrosion of aluminum produces a thin oxide film that shields further damage. This process protects aluminum against any severe kind of corrosion and thus makes the metal very durable in most situations.
Defining Rust vs. Corrosion
| Parameter | Rust | Corrosion |
|---|---|---|
| Definition | Oxidation of iron or steel | Degradation of materials due to chemical reactions |
| Occurs in | Only iron or steel | Metals, ceramics, and polymers |
| Cause | Exposure to oxygen and moisture | Chemical reactions with environment |
| Common Appearance | Red, flaky material | Varies depending on the material |
| Protective Layer Formed | No, rust deteriorates material | Sometimes, forms protective oxide (e.g., aluminum) |
| Speed of Process | Slower compared to general corrosion | Varies; can be fast or slow |
| Material Affected | Specific to ferrous metals | Broad range of materials |
| Environmental Factors Required | Moisture and oxygen | Can occur in liquids, gases, or moist air |
| Prevention Methods | Coatings, galvanization, alloys | Varies by material and environmental exposure |
Why Aluminum Doesn’t Rust
Due to its unique chemical and structural properties, aluminum gets protected from rusting. Aluminum essentially undergoes passivation. In the air, aluminum immediately absorbs oxygen from its surrounding atmosphere, which results in formation of a thin layer of aluminum oxide. This oxide is tightly bonded to the surface and provides several benefits:
- Continuous, non-porous barrier: Acts as a shield against moisture, salts, and other contaminants
- Chemical inertness: Aluminum oxide is chemically stable and unreactive
- Enhanced hardness: Greater hardness than base aluminum, providing better wear resistance
- Self-healing properties: The layer can regenerate if damaged
The Protective Oxide Layer of Aluminum
Once aluminum is exposed to oxygen in the air, its surface develops a thin film of aluminum oxide (Al₂O₃) through passivation. This layer, measuring only a few nanometers in thickness, is nonetheless very effective in protecting against further oxidation.
Scientific Insight: Aluminum oxide has exceptional durability due to its high enthalpy of formation and superior ionic bonding, making it resistant to various chemical and physical environments.
Enhanced Protection Through Anodizing
The properties of the native oxide can be greatly enhanced by anodizing, which thickens and smoothens the oxide layer. Anodized aluminum finds uses in applications requiring high abrasion resistance and attractive finishes, including:
- Electronics industry
- Automotive applications
- Architectural industries
- Capacitor and semiconductor device manufacture
Factors That Affect Aluminum’s Corrosion Resistance

- Surface Treatments: Anodizing and coating provide high corrosion resistance by forming environmental barriers
- Alloy Composition: Alloying elements like magnesium or silicon influence corrosion resistance; high purity alloys usually perform better
- Environmental Conditions: High humidity, saline environments, and acidic/alkaline solutions encourage corrosion without surface protection
- Surface Contaminants: Dirt, grease, or metallic particles may cause localized attack leading to pitting corrosion
- Mechanical Damage: Scratches and dents can remove protective layers, depleting aluminum’s corrosion resistance
Environmental Influences on Corrosion
Environmental situations govern the corrosion activity of materials, including aluminum. Key influential variables include:
Primary Environmental Factors
- Humidity and moisture: High moisture environments accelerate corrosion processes
- Temperature fluctuations: Thermal expansion and contraction cycles create stresses and microcracks
- Atmospheric pollutants: Industrial pollution containing SO₂ and NOₓ creates acidic environments
- Coastal environments: High saline content in air accelerates corrosion through chloride interactions
Key Strategy: Understanding the interaction between environmental factors and material performance is crucial for successful mitigation. Protective coatings, alloy selection, and controlling exposure environment are top strategies for enhancing aluminum’s resistance to environmental corrosion.
Alloy Composition and Corrosion Resistance
The corrosion resistance of aluminum alloys depends heavily on chemical composition and microstructure. Here’s how different alloy series perform:
| Alloy Series | Main Alloying Element | Corrosion Resistance | Key Characteristics |
|---|---|---|---|
| 1xxx Series | Pure aluminum | Excellent in most environments | Good corrosion resistance but limited mechanical strength |
| 2xxx Series | Copper | Susceptible to pitting | High strength but vulnerable to stress corrosion cracking |
| 5xxx Series | Magnesium | Good in marine environments | Forms passivating oxide layer but vulnerable to intergranular corrosion at elevated temperatures |
Recent advances in metallurgical engineering show that optimization of alloying elements combined with thermomechanical treatments can improve both corrosion resistance and mechanical performance simultaneously.
Practical Steps to Prevent or Minimize Aluminum Corrosion
- Apply Protective Coatings: Use anodizing, painting, or powder coating methods as barriers against corrosion
- Ensure Proper Drainage: Design structures to allow water and moisture to drain, preventing liquid accumulation
- Use Corrosion-Resistant Alloys: Select aluminum grades suitable for high moisture, salt, or chemical exposures
- Avoid Galvanic Corrosion: Prevent aluminum attachment to other metals in electrolyte presence using insulating materials
- Apply Corrosion Inhibitors: Use chemical treatments like chromate or phosphate conversion coatings
- Control Environmental Exposure: Limit exposure to saltwater, industrial pollutants, or acid rain through positioning or enclosure
Regular Cleaning and Maintenance Tips
Routine Cleaning Protocol
- Clean regularly with gentle detergent and water using soft cloth or sponge
- Avoid abrasive pads or metal tools that can damage aluminum surfaces
- Regular cleaning can reduce surface pitting chances by up to 25%
Inspection Guidelines
- Perform periodic inspections for corrosion indicators: discoloration, flaking, or crystalline powder deposition
- High-moisture, saline environments require visual inspection every two months
- Focus on critical areas demanding prompt intervention
Protective Coating Maintenance
- Refinish protective coatings as needed (anodizing, powder coating, clear lacquer)
- Well-maintained anodized surfaces can increase component service life up to 40% in harsh conditions
Best Practices
- Use Neutral pH Cleaning Agents: Avoid acidic or strongly alkaline cleaners that damage aluminum surfaces
- Rinse Thoroughly: Remove residual cleaning solutions with potable water immediately after cleaning
- Dry Properly: Use lint-free microfiber cloths, paying attention to crevices where moisture accumulates
Coatings and Sealants for Enhanced Protection
Advanced coating systems provide aluminum with superior environmental protection and corrosion resistance. Modern options include:
Advanced Coating Technologies
- Anodizing: Electrochemical conversion enhancing natural oxide thickness beyond 1 µm
- Powder Coating: Provides barrier against moisture with impact and abrasion resistance
- Nanotechnology Sealants: Create hydrophobic and oleophobic surfaces that repel water and dirt
Sealant Applications
- Fill minor gaps, crevices, or coating joints where moisture could penetrate
- Provide UV radiation protection preventing lengthy exposure degradation
- Reduce maintenance requirements and extend aluminum component lifetime
Anodizing vs. Powder Coating for Aluminum
| Key Point | Anodizing | Powder Coating |
|---|---|---|
| Process Type | Electrochemical oxidation | Electrostatic powder application |
| Layer Thickness | Typically 5–25 microns | Typically 60–80 microns |
| Color Options | Limited, often natural metallic hues | Wide range of colors and finishes |
| Corrosion Resistance | High, especially in marine environments | High, but depends on coating quality |
| UV Resistance | Good for prolonged exposure | Excellent with UV-stable coatings |
| Durability | Highly scratch-resistant | Impact and abrasion-resistant |
| Surface Appearance | Semi-gloss, natural metallic finish | Glossy, matte, or textured finishes |
| Eco-Friendliness | Minimal chemical waste | Produces overspray, recyclable material |
| Cost | Moderate | Generally higher |
| Maintenance Requirements | Low, regular cleaning suffices | Low, with occasional touch-ups |
Summary of Aluminum Corrosion Insights

Recent advances have improved understanding of aluminum corrosion behavior across different environmental conditions. Key findings include:
- Natural Protection: Aluminum builds a thin protective metallic oxide film providing excellent natural corrosion resistance
- Environmental Factors: Layer susceptibility depends on pH, temperature, and local chloride ion concentration
- High-Risk Environments: High salinity coastal zones show high pitting corrosion potential, while industrial pollutants accelerate uniform surface degradation
- Microstructural Influence: Grain size affects corrosion susceptibility, with alloying elements like magnesium or silicon playing roles in electrochemical stability
- Advanced Analysis: Modern techniques like electrochemical impedance spectroscopy (EIS) and scanning electron microscopy (SEM) enable detailed corrosion mechanism examination
Optimization Strategy: Extending aluminum material life expectancy requires intelligent material selection combined with comprehensive environmental assessments and appropriate protective measures including anodizing, surface coatings, and inhibitors.
Final Advice for Maintaining Aluminum Products
For optimal aluminum product performance, implement these evidence-based maintenance practices:
- Regular Cleaning: Use non-abrasive materials to prevent corrosive agent accumulation
- Protective Layers: Apply anodizing or powder coatings as barriers against oxidation and UV exposure
- Structural Inspections: Conduct regular examinations for fatigue, stress corrosion, or micro-cracks
- Joint Design: Use proper sealing techniques to reduce galvanic corrosion when aluminum contacts dissimilar metals
- Preventive Measures: Adapt maintenance strategies to operational environments for optimal reliability and minimal lifecycle costs
Reference Sources
-
Research on Properties and Applications of New Lightweight Aluminum Alloy Materials
- Key Findings: This study explores the properties of 5052 aluminum alloy, which is widely used for its anti-corrosion properties. The alloy’s high magnesium content enhances its resistance to environmental degradation, making it suitable for various applications.
- Read more
-
Effect of Anodizing on Aluminum Alloy 2024 with Boric Sulfate Acid in Medium 3.5% NaCl
- Key Findings: This study examines the impact of anodizing aluminum alloy 2024 in a boric sulfate acid medium. It highlights how anodizing improves the alloy’s resistance to corrosion in saline environments.
- Read more
Frequently Asked Questions (FAQs)
Q: Does aluminum corrode like iron and steel?
A: No, aluminum does not rust like iron or steel. Rust refers to iron corrosion occurring with moisture and oxygen exposure. Aluminum undergoes aluminum oxidation, forming a thin protective oxide layer that prevents further oxidation. However, aluminum can corrode under certain conditions, especially in wet environments or when exposed to chlorides and sulfides.
Q: What is the aluminum oxidation process?
A: The aluminum oxidation process occurs when aluminum reacts with oxygen to form aluminum oxide. This reaction happens when aluminum is exposed to moisture or air oxygen. The oxide layer forms a hard, protective barrier preventing underlying metal corrosion. If damaged, aluminum atoms become exposed and aluminum will corrode, with rates increasing in harsh conditions like salt water.
Q: How can I prevent corrosion in aluminum?
A: Prevent aluminum corrosion by maintaining protective oxide layer integrity. Avoid exposure to harsh chemicals and environments like salt water or acidic conditions. Use sacrificial anodes for aluminum structures, apply protective coatings or paints, and conduct regular maintenance and inspections to identify oxide layer damage for prompt repairs.
Q: Does aluminum rust in salt water?
A: Aluminum doesn’t rust traditionally but can corrode in salt water. Saltwater environments increase galvanic corrosion risk, particularly when aluminum contacts iron-containing metals. Corrosion involves aluminum reacting with oxygen and salt to form damaging compounds. Use sacrificial anodes to protect aluminum structures in marine environments.
Q: What happens to aluminum when exposed to moisture?
A: When exposed to moisture, aluminum undergoes oxidation, forming a beneficial surface oxide layer that protects underlying aluminum from further corrosion. However, if the protective layer is compromised, aluminum can corrode, especially if moisture contains salts or acids. Corrosion rates depend on environmental conditions, with wet and salty conditions accelerating the process.






